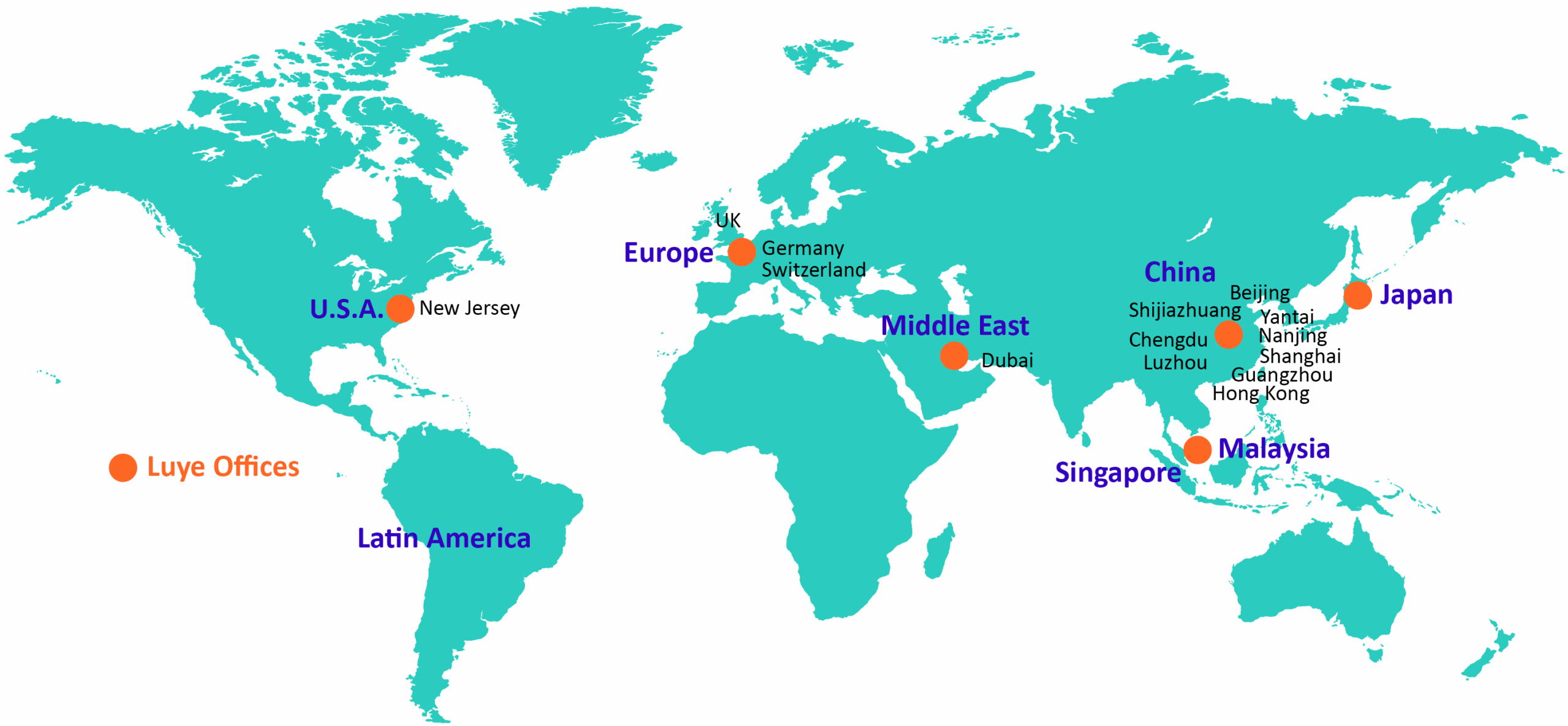
Luye has established R&D centers in the United States, Europe, and China.
Luye has received approval for 16 innovative products in multiple global markets since 2021. Among these, ERZOFRI® (paliperidone palmitate)* received FDA approval, and its launch in 2025 served as a foundation for the establishment of the Luye Pharma USA market.1
- ERZOFRI® carries a BOXED WARNING and is not approved for use in patients with dementia-related psychosis. See full Prescribing Information and complete BOXED WARNING by clicking here: ERZOFRI® Prescribing Information.
most respected global
leaders in the life sciences
industry
Philosophy
Operational Excellence
Employee Development
Cooperation
Innovation
Excellence
Our growth and acquisitions
Luye Pharma’s timeline of growth
In 2016, Luye Pharma Group acquired the transdermal drug delivery system business from the Swiss pharmaceutical company Acino, expanding operations to major international markets including Europe, the United States, and Japan.
Luye Pharma USA was established in 2015 and entered the US commercial market in 2025 with the launch of ERZOFRI® (paliperidone palmitate).1

We are looking to expand our portfolio
For more information on license acquisition and what it means to partner with Luye Pharma USA, click below.
Find out moreReference: 1. ERZOFRI® [Prescribing Information]. Princeton, NJ: Luye Pharma Group Ltd.